ERT AM3
Make home monitoring easy with our integrated home spirometer and electronic diary
ERT AM3
Make home monitoring easy with our integrated home spirometer and electronic diary

Related to recommend
EASY-TO-USE HOME SPIROMETERAND eDIARY
The ERT AM3TM is built using the proven JAEGERTM Asthma MonitorTM and is the first home spirometer and eDiary to receive Asthma Control Questionnaire (ACQ) certifications. This easy‑to‑use device measures and saves all relevant expiratory flow‑volume parameters, such as PEF and FEV1. The recording of symptoms, events and medication, including severity and dose, make the AM3 a complete electronic diary (eDiary) for patient home monitoring. The AM3 supports compliance by guiding the patient through the assessment with clear, easy‑ to‑read, on‑screen instructions. Additionally, the device
utilizes powerful branching logic to ensure patients answer the appropriate questions and provides automatic scoring to give patients immediate feedback.
The AM3 with optional mobile communications functionality wirelessly transfers data automatically after a session or scheduled at a fixed time of day via an internal global cellular modem using economic text messaging. This ensures data
is simply and cost‑effectively transferred from a patient’s home or any remote location to your investigative site and our EXPERT platform, enabling near real‑time access to patient data. An analog modem solution is also available for patients without network coverage.
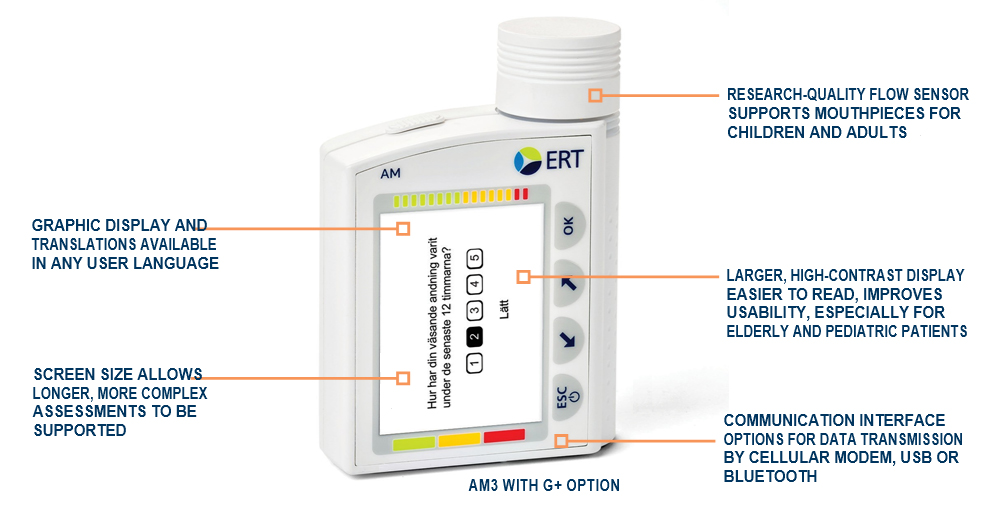
AM3 FEATURES
> Spirometry compliant with ATS/ERS 2005 standards
> Research‑quality flow sensor supports mouthpieces for children and adults
> Graphic display and translations available in any user language
> Automated date/time stamped records available
> Non‑volatile memory stores months of spirometry and eDiary data
> Bluetooth® reduces site burden by wirelessly connecting an AM3 to ERT MasterScope, eCOA Tablet, AMOS software and other devices
![]() TECHNICAL
SPECIFICATIONS
TECHNICAL
SPECIFICATIONS
|
AM3/AM3 BLUETOOTH |
|
|
Volume |
0.5 to 8 L |
|
Flow |
0 to 840 L/min |
|
Capacity |
1200 measurements and 400 sets of diaries (8000 answers) |
|
Type |
Secure, non‑volatile memory, no backup required |
|
L x W x H |
112 mm x 82 mm x 37 mm |
|
Weight |
167 g (includes batteries) |
|
Screen resolution |
4.7 x 3 cm or 2.2” active area |
|
Battery |
3 x 1.5V (AAA, included) |
|
Backlight |
White LED |
|
Communications |
Serial, USB and Bluetooth (BT) |
|
AM3 WITH GSM OPTION |
|
|
Weight |
120 g (includes battery) |
|
Battery |
Built‑in rechargeable Li‑Ion polymer battery |
|
Regulations |
FCC/IC certified |
|
Communications |
Serial, USB, GSM and Bluetooth (BT) optional |
|
AM3 WITH G+ OPTION |
|
|
Weight |
150 g (includes battery) |
|
Screen Resolution |
5.7 x 4.3 cm or 2.8” active area |
|
Battery |
Built‑in rechargeable Li‑Ion polymer battery |
|
Regulations |
FCC/IC certified |
|
Communications |
USB, 3G and Bluetooth |
|
OPTIONAL ACCESSORIES |
|
Rotary flow sensor replacements |
|
Additional mouthpieces |
|
Sniff option to measure nasal PIF |