
ERT RESPIRATORY SOLUTIONS
Demand data quality and breathe easy in your clinical trials
IMPROVE THE QUALITY OF YOUR RESPIRATORY TRIAL DATA
Managing risk and generating high quality respiratory data is notably difficult in clinical trials. Complex protocols, lack of training and collection and analysis inconsistencies create uncertainty about patient eligibility, safety and compound efficacy.
You need greater confidence in the quality of respiratory data to remove any uncertainty about the accuracy and acceptability of data. To that end, centralized spirometry and other pulmonary function tests (PFTs) have become irreplaceable diagnostic, screening and data collection tools for pulmonary and other lung safety or disease progression clinical trials.
PROVEN SOLUTIONS FOR CENTRALIZED SPIROMETRY AND PFT
With diagnostic spirometry and PFT, you can optimize pulmonary function data quality for better patient and site performance, increased protocol compliance and reduced investigative site burden. But with so many solutions available, how do you know what’s right for your trial?
Our integrated respiratory solutions apply leading technology, unrivalled expertise, global resources and a centralized approach to deliver the highest quality data for your global clinical trials.
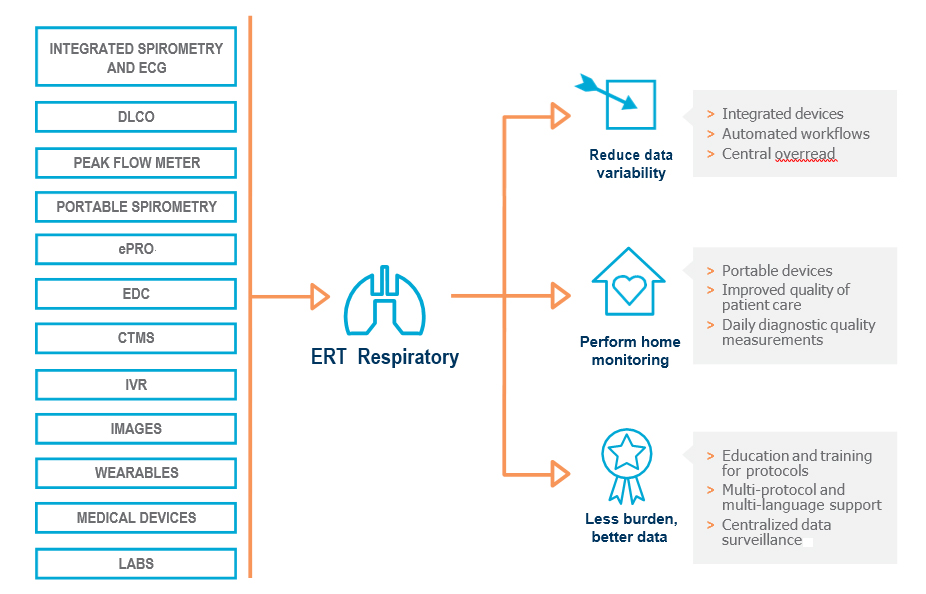
ERT RESPIRATORY: CENTRALIZED APPROACH FOR HIGH-QUALITY DATA
Reduce data variability and integrate home monitoring solutions for less burden on sites and better data
IMPROVE PULMONARY FUNCTION DATA QUALITY
Drive high‑quality data with our integrated diagnostic spirometry and PFT solution.
Control workflows for compliance and quality results
Software customization and standardized devices that meet ATS and ERS guidelines help you enforce standards and protocols.
Reduce site burden and variabilitySpecialized site training, technology and global support reduces site burden and data variability.
Increase your respiratory statistical power
Central quality review of site data drives lower costs for reliable efficacy and safety data.
Drive the capture of nearly 100% acceptable data
Acceptability and best test review of patient efforts by our pulmonary specialists protects your investment and augments data quality with consistent site feedback.Identify and mitigate risks
Centralized, real‑time visibility empowers quality risk management (QRM) and risk‑basedmonitoring (RBM) strategies so you can recognize and reduce risks for every site and patient.
