ERT MASTERSCOPE
Minimize risk and uncertainty with our centralized spirometry,ECG, patient reported outcomes and multi-endpoint platform

ERT MASTERSCOPE
Minimize risk and uncertainty with our centralized spirometry,ECG, patient reported outcomes and multi-endpoint platform

Related to recommend
The ERT MasterScopeTM helps you overcome the challenges of working with various diagnostic technologies while following a complex clinical trial protocol. MasterScope’s highly intuitive multi-language user interface, customized protocol workflows and many smart features such as biometric user identification (21 CFR Part 11 compliance) make it the preferred choice for your centralized spirometry studies. With a unique multi-protocol device reuse option, MasterScope also minimizes equipment costs and logistics, speeds trial start-up and eases investigative site burden.
MasterScope’s integrated platform helps your sites to better focus on patient safety and data quality.
Built upon the proven JAEGERTM pneumotach sensor, MasterScope offers complete spirometry capabilities (exceeding current ATS/ERS standards), integrates home monitoring and patient reported outcomes eDiary data from our AM3TM device family and offers an optional
12-lead digital ECG utilizing the ERT HESTM algorithm. With third party data integration like the Circassia NIOX VERO® for collecting exhaled nitric oxide (FeNO) and other advanced features, MasterScope will become your central diagnostic workstation.
> Integrate Spirometry, 12-lead ECG, FeNO and home spirometric monitoring with patient reported
outcomes (PROs) eDiary data in one high performance system
> Simplify the most complex of trials with customizable, protocol-specific workflows
> Minimize equipment costs and logistics with a unique multi-protocol option that allows one device to support multiple studies
> Drive site performance with software designed with site needs in mind, including multi-language support

> Spirometry according to ATS/ERS standards
> Supports Quanjer-Global Lungs Initiative (GLI)-2012 reference values
> Automated error detection:
• Back extrapolated volume
• Late peak flow
• Coughing
• Expiration time <6 s (<3 s for children), including graphical visualization during measurement
• “No plateau detection” at the end of expiration, including graphical visualization during measurement
> Automated acceptability and repeatability checks, including visualization by traffic light indicator
> Enforced resting time for the patients between measurements
> Volume/time and Tiffeneau views
> Smart control for manual selection and deselection of loops by the user, including user comments (deselected loops will be stored, transmitted and reviewed by ERT’s pulmonary overread experts)
> Automated scoring and alerting calculation (e.g. FEV1 variation)
> Automated calculation of inclusion, randomization, exclusion or alert criteria
> Waiting Room function for easy multi-subject management on a single visit day
> eLearning with local and online training videos and documentation
> User verification by biometric fingerprint detection
> Electronic submission and management of data correction requests
> Protocol amendments and new features deployed via automatic online updates
> Secure Ethernet (LAN), wireless (WLAN), SD card web upload or analog data transmission
> Fully functional offline operation
> Online setup for initial protocol software setup
> Connects to our EXPERT® platform for centralized overread with optional data access and analytics
> Online portal for near real-time data management and reporting
Drive high quality spirometry data
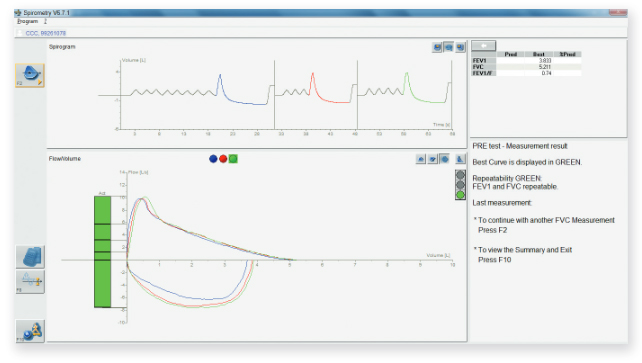
MasterScope’s advanced logic delivers the highest quality spirometry data. An intelligent traffic light indication for automated acceptability and repeatability checks provides site operators with immediate feedback on data quality. Other smart features, such as control of the resting time between maneuvers, help to achieve the best spirometry results from each patient.
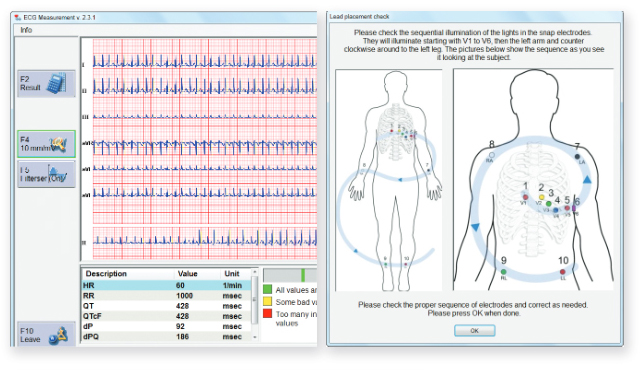
Capturing the highest quality safety data on time and in the correct format is critical for new drug approvals. MasterScope integrates an optional solution to centrally collect, interpret and distribute ECG data. To ensure proper placement of ECG electrodes, LEDs guide
users by flashing in the appropriate sequence, a unique feature. Prior to testing, MasterScope also automatically checks the connectivity of each lead to ensure the best quality signal.

MasterScope is the only available diagnostic platform that provides biometric fingerprint identification for site operators in compliance with 21 CFR Part 11 requirements — no passwords are required. The system also automatically creates a comprehensive audit trail log, simplifying quality and compliance monitoring.

With MasterScope, you can offer site users a software interface in their native language to boost their efficiency. User-friendly
features, such as a waiting room function, make it easier to manage multiple patients on the same visit day. This enables site users to view a dashboard that includes alerts on when the next action needs to be performed by each patient (e.g., the next serial spirometry measurement). Users can also seamlessly switch between patient profiles with a single click.
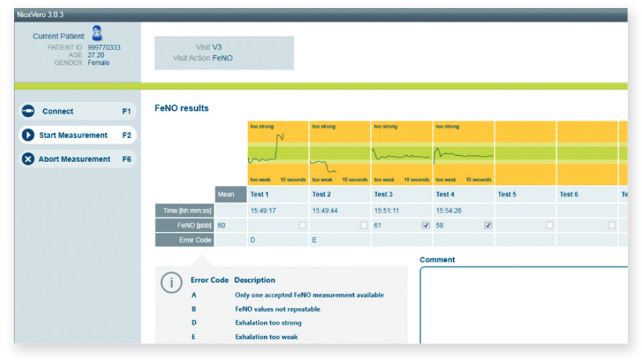
If your protocol requires collection of additional parameters such as home monitoring with patient reported outcomes eDiaries or fractional exhaled nitric oxide (FeNO), we offer fully integrated solutions that visualize and centralize such data. MasterScope also completes regular automated quality checks, reviews calibration efforts and automatically updates software to ensure the latest protocol amendments and features are being used at each site, reducing burden on site personnel while increasing trial efficiency.
|
SPIROMETRY SPECIFICATIONS |
|
|
Flow measurement |
High quality pneumotach |
|
Flow range |
0.1 to 16 L/s |
|
Flow accuracy |
0.1 to 14 L/s: +/-5%/0.2 L/s |
|
Flow resolution |
10 mL/s |
|
Resistance |
0.05 kPa/(L/s) at 10 L/s |
|
Volume measurement |
Digital integration |
|
Volume range |
0.1 to 8 L |
|
Volume accuracy |
0.5 to 8 L: +/-3%/0.05 L |
|
Volume resolution |
1 mL |
|
ECG SPECIFICATIONS |
|
|
Active contact test |
LED indicator snap |
|
Sampling rate |
1000 Hz per channel |
|
Pacemaker detection |
4000 Hz sampling, detection in amplifier |
|
Resolution |
2.6 uV/bit ECG, 19 bit |
|
Interpretation |
Hannover ECG System (“HES”) |
|
OPTIONAL DEVICES |
|
12-lead digital ECG |
|
AM3™ electronic PEF/FEV1 meter and diary |
|
Circassia NIOX VERO® |
|
OPTIONAL ACCESSORIES |
|
Bacterial filters |
|
Nose clips with nose pads |
|
Pneumotach sensors |
|
ECG electrodes |